The United States has made significant strides in the battle against COVID-19, with the Food and Drug Administration (FDA) authorizing the first over-the-counter at-home rapid test that delivers immediate results without the need for a prescription or lab processing. This milestone could accelerate testing accessibility not only in the U.S. but potentially influence global testing strategies, including in Asia.
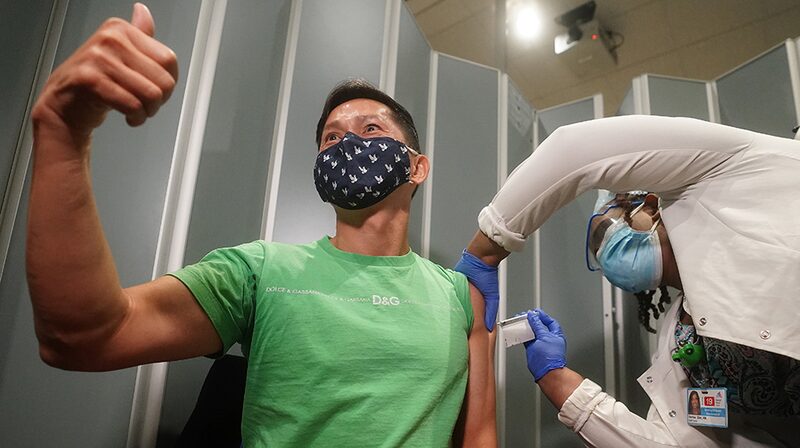
In addition to advancements in testing, the FDA is expected to approve a second COVID-19 vaccine developed by Moderna by the end of the week. A recent review by FDA scientists found the vaccine to be highly effective, demonstrating a 94% efficacy rate during clinical trials, with no serious safety concerns. The vaccine’s success across diverse age groups, races, and genders offers hope for widespread immunization efforts.
According to reports, approximately 20 million Americans could be immunized by the end of December. Major pharmacy chains like CVS and Walgreens are collaborating with the federal government to facilitate vaccinations, prioritizing healthcare workers and residents and staff of long-term care facilities.
While these developments represent a significant breakthrough, challenges remain. Vaccines may not be readily available to the general public for some time, and infection rates continue to climb, with hospitals across the U.S. reaching capacity. The progress in the U.S. is closely watched by countries worldwide, including those in Asia, as global cooperation and vaccine distribution are crucial in combating the pandemic universally.
Reference(s):
COVID-19: U.S. makes headway with vaccines & at-home testing
cgtn.com