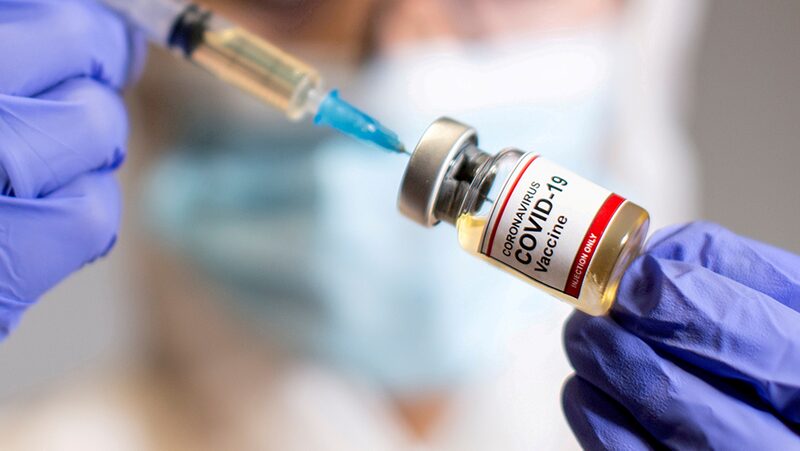
As the United States grapples with a surge in COVID-19 cases, preparations are underway for the distribution of vaccines developed by Moderna and Pfizer. Both companies have requested emergency use authorization from the U.S. Food and Drug Administration (FDA), raising hopes that the first batches of vaccines could be ready by mid to late December.
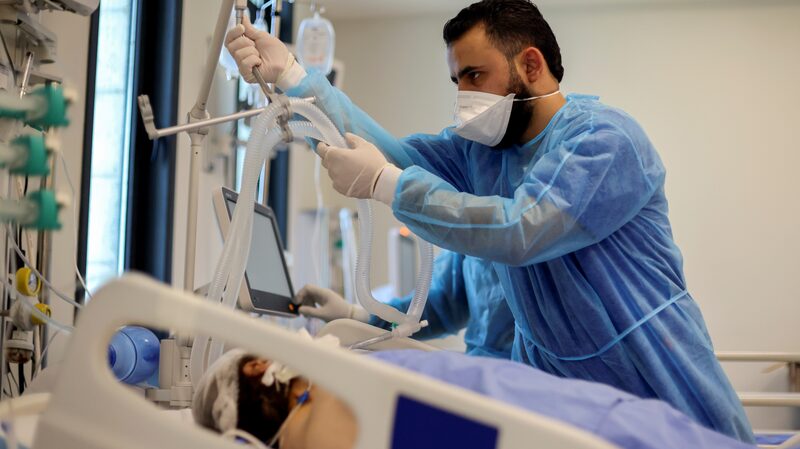
Health officials caution that despite the promising developments, the nation faces a challenging winter ahead. New infections continue to rise across the country, and widespread vaccine availability is still months away. Dr. Anthony Fauci, a leading infectious disease expert, has indicated that a COVID-19 vaccine may not be widely accessible to the general public until April or May 2021.
On Tuesday, an advisory panel from the Centers for Disease Control and Prevention (CDC) is set to meet to determine the prioritization for vaccine distribution. It is expected that healthcare workers and residents of nursing homes will be among the first to receive immunizations.
Following the advisory group’s recommendations, approvals will be needed from the CDC Director and the Secretary of Health and Human Services. Once approved, these guidelines will become official CDC guidance. States are required to submit their initial vaccine distribution plans to the federal government by December 4th, outlining how they will manage the allocation of the first vaccine doses.
The imminent rollout of vaccines brings a sense of optimism, but officials emphasize the importance of continued public health measures. Masks, social distancing, and hand hygiene remain critical in controlling the spread of the virus until vaccines become widely available.
Reference(s):
cgtn.com