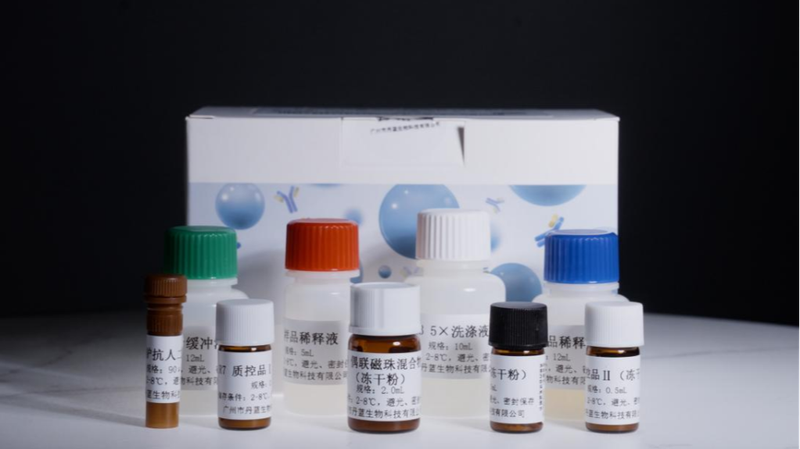
Chinese medical researchers have achieved a major milestone in cancer diagnostics with the approval of a novel lung cancer detection kit by the National Medical Products Administration. Developed by the Hangzhou Institute of Medicine under the Chinese Academy of Sciences, this Class III medical device uses innovative biomarker technology to identify early-stage lung cancer through blood tests.
Lung cancer remains the leading cause of cancer deaths in China, with most cases detected too late for effective treatment. The new kit addresses critical gaps in existing screening methods by analyzing tumor autoantibodies – immune system markers that appear during cancer's earliest molecular stages.
"Our technology acts like a biological alarm system," explained lead researcher Hu Hai. "It detects cancer signals before symptoms appear or tumors become visible on scans, giving patients a crucial time advantage."
The development team employed artificial intelligence to analyze hundreds of cancer-related proteins, ultimately identifying 13 key biomarkers – eight of which represent new scientific discoveries. Clinical trials across major hospitals demonstrated 65% sensitivity in detecting early-stage cancers, significantly outperforming traditional diagnostic methods.
This innovation comes as China intensifies efforts to improve cancer survival rates through its Healthy China 2030 initiative. Medical experts predict the test could become standard in primary care settings and routine health screenings, particularly for high-risk groups like long-term smokers.
While low-dose CT scans remain important for lung cancer screening, the new blood-based method offers complementary advantages. It requires no specialized imaging equipment and could help reduce unnecessary follow-up procedures for benign nodules detected through CT scans.
The kit's approval marks a significant step forward in China's growing medical technology sector, with potential implications for global cancer screening protocols. Researchers plan to expand clinical applications while exploring adaptations for other cancer types.
Reference(s):
New kit for lung cancer early detection approved for market in China
cgtn.com