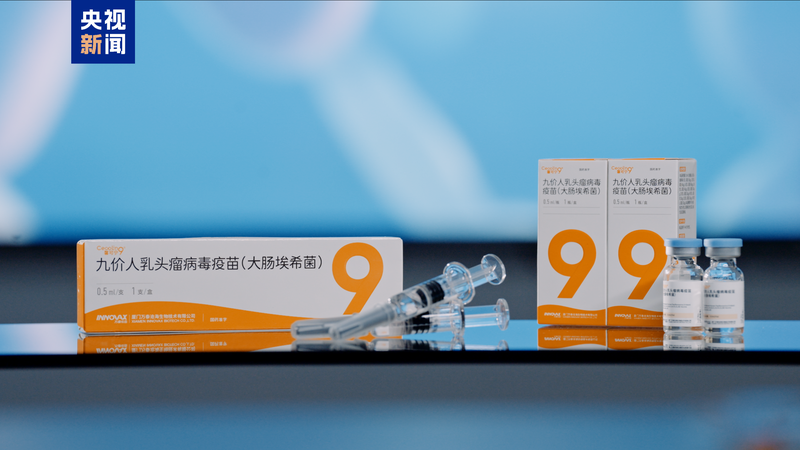
China's National Medical Products Administration approved the country's first domestically developed nine-valent human papillomavirus (HPV) vaccine on Wednesday, marking a milestone in public health innovation. The vaccine, named Cecolin 9, was jointly created by the Xiang An Biomedicine Laboratory, Xiamen University, and Wantai BioPharm.
This breakthrough positions China as the second country globally – after the United States – capable of independently producing high-valency HPV vaccines. Clinical trials spanning five years involved over 11,000 volunteers aged 9-45, demonstrating 98% effectiveness against persistent HPV infections and complete protection against cervical infections from targeted strains.
Notably, the vaccine requires only two doses for girls aged 9-17, matching the immune response of three doses in adults. For adolescents aged 15-17, it becomes China's sole two-dose HPV vaccine option. Published in The Lancet Infectious Diseases, trial data shows comparable long-term efficacy to international counterparts for at least 30 months post-vaccination.
The development team previously made history with China's first domestic two-valent HPV vaccine in 2019, which later achieved WHO pre-qualification and distribution in 21 countries. This advancement comes as cervical cancer remains the fourth most common cancer among women worldwide, with China expanding free HPV vaccination to 40% of 13-14-year-old girls in 2024.
Reference(s):
China approves first domestically produced nine-valent HPV vaccine
cgtn.com