Chinese researchers have developed a groundbreaking self-cleaning electrode that significantly enhances the stable synthesis of alkaline-earth metal peroxides (MO2), offering promising applications in environmental and industrial processes.
The team, from the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences and Shanghai Jiaotong University, published their findings in the journal Nature Nanotechnology on Monday.
MO2, an alternative to hydrogen peroxide, boasts excellent oxidative properties, superior chemical stability, high purity, and ease of storage and transportation. It has been widely utilized in wastewater treatment and disinfection. However, traditional synthesis methods of MO2 involve rapid decomposition of hydrogen peroxide, leading to inefficient utilization and increased risks during storage and transport.
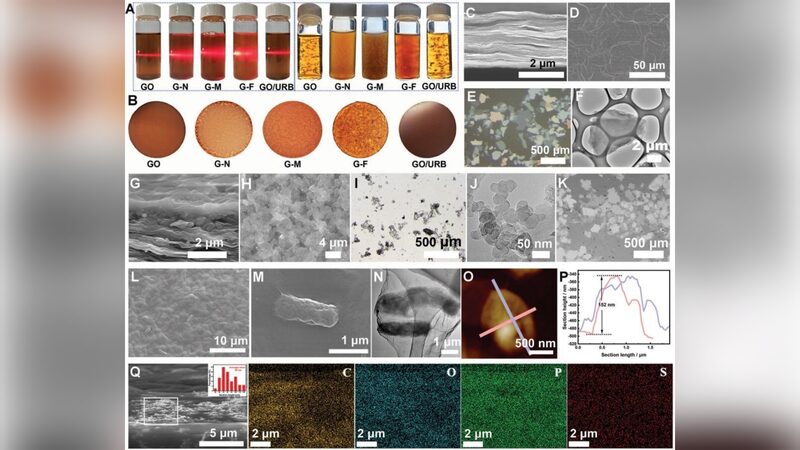
To address these challenges, the researchers proposed an in-situ electrochemical synthesis process for MO2. Central to this innovation is the development of a nickel-doped oxygenated carbon electrode with a Teflon coating, featuring a micro-nanostructure and low surface energy. This design effectively reduces the severe adhesion of solid MO2 products on the electrode surface.
“The carbon electrode greatly reduces the solid-liquid contact area with the electrolyte, facilitating rapid detachment of the in-situ generated MO2 from the self-cleaning electrode surface,” explained Professor Lu Zhiyi of NIMTE.
The Teflon-coated electrode demonstrated remarkable stability, operating continuously for over 1,000 hours at a current density of 50 milliamperes per square centimeter during the electrochemical synthesis of MO2. This longevity suggests broad application potential for the technology in industrial settings.
The innovative self-cleaning electrode not only enhances the efficiency and stability of MO2 production but also mitigates economic losses and reduces explosion risks associated with hydrogen peroxide storage and transportation, marking a significant advancement in the field of electrochemical synthesis.
Reference(s):
cgtn.com