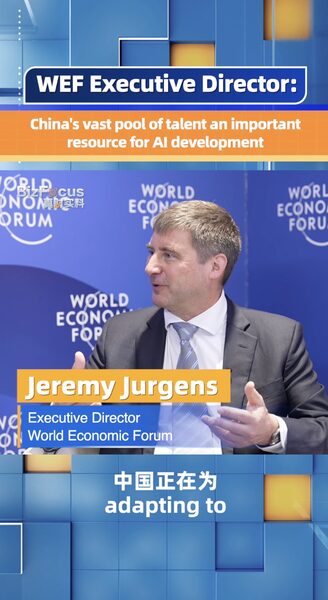
Global pharmaceutical giant AstraZeneca is set to launch over 100 new medicines in China within the next five years, leveraging its Research and Development (R&D) center in Shanghai to drive innovation. Sharon Barr, Executive Vice President of Biopharmaceuticals R&D at AstraZeneca, highlighted the company’s commitment to mutual collaboration with Chinese partners during an interview with CGTN.
“Our R&D center in Shanghai plays a pivotal role in our global innovation strategy,” Barr stated. “By working closely with local collaborators, we aim to address pressing healthcare needs and bring transformative medicines to patients in China and around the world.”
The planned introduction of new medicines underscores AstraZeneca’s dedication to the Chinese market, focusing on therapeutic areas such as oncology, cardiovascular, renal, and metabolic diseases. Barr emphasized that the synergy between AstraZeneca and its Chinese collaborators is crucial for accelerating the development of new treatments.
“China’s dynamic healthcare landscape offers immense opportunities for innovation,” she said. “Through collaboration, we can harness the strengths of both AstraZeneca and our Chinese partners to achieve shared goals and improve patient outcomes.”
The company’s strategic investments in China align with the country’s emphasis on healthcare innovation and development. The Shanghai R&D center serves as a hub for cutting-edge research, fostering partnerships with local institutions and contributing to the global pharmaceutical industry.
Industry analysts view AstraZeneca’s initiative as a significant step in enhancing international cooperation in biotech and pharmaceuticals within Asia. The move is expected to stimulate growth, inspire technological advancements, and ultimately benefit patients seeking new and effective treatments.
Reference(s):
AstraZeneca EVP: Explore mutual interests with Chinese collaborators
cgtn.com