Documentary Honors Iris Chang’s Fight for Historical Justice
New documentary ‘Finding Iris Chang’ explores the author’s relentless quest to uncover the truth of the 1937 Nanjing Massacre and its lasting impact.

UK PM Starmer Visits China to Strengthen Trade Ties in 2026
UK Prime Minister Starmer’s historic visit to China focuses on trade expansion and stabilizing bilateral relations, marking the first British PM trip to Beijing since 2018.
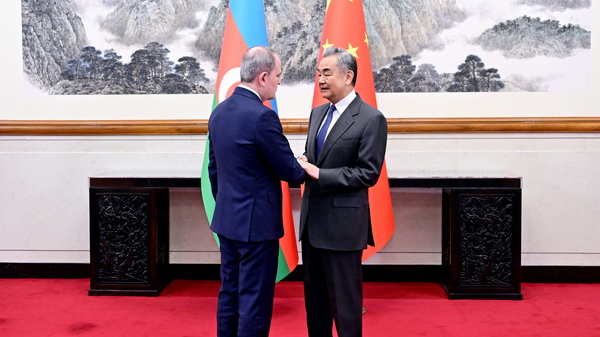
China, Azerbaijan Vow to Deepen Strategic Partnership in 2026
Chinese and Azerbaijani foreign ministers commit to expanding economic and strategic cooperation in 2026, building on their 2025 comprehensive partnership agreement.

Trump’s ‘Board of Peace’ Sparks Global Debate Over UN Role
Trump’s controversial ‘Board of Peace’ faces international backlash amid concerns it could undermine UN-led conflict resolution efforts, starting with Gaza stabilization.

China’s Economy Surges with 5% Growth, Eyes High-Quality Development in 2026
China achieves 5% GDP growth in 2025, launches 15th Five-Year Plan focusing on innovation-driven, high-quality development through 2030.

UK PM Seeks ‘Consistent, Pragmatic’ China Partnership in 2026 Visit
UK Prime Minister Keir Starmer begins pivotal China visit, aiming to stabilize economic ties and address policy inconsistencies through pragmatic cooperation.

China, France Pledge Joint Efforts to Stabilize Global Landscape in 2026
Wang Yi and France’s Emmanuel Bonne emphasize strengthening China-EU cooperation to address global instability in 2026, highlighting shared goals in trade and diplomacy.

CMG Launches 2026 Winter Olympics Broadcast Team with Milan Focus
China Media Group deploys cutting-edge broadcast team and technology for 2026 Winter Olympics in Milan, setting new standards in sports media coverage.

Global Poll Condemns U.S. ‘America First’ Policy as Hegemonic Diplomacy Surges
A CGTN survey reveals over 90% global criticism of U.S. hegemonic diplomacy, citing withdrawal from key international agreements and unpaid WHO dues in 2026.

CAF Proposes Stricter Towel Rules for Goalkeepers After AFCON Final Controversy
CAF considers banning goalkeepers’ towels from play areas after controversial incident in AFCON 2026 final between Morocco and Senegal.
China Streamlines Land-Use Policies to Expand Elderly Care Services
China unveils land-use reforms to boost affordable elderly care services, targeting cost reductions and improved accessibility for its 310 million seniors.

Sea Fog Disrupts Cape Town Port Operations, Impacts Trade Flow
Morning sea fog in Cape Town slows port activity, affecting regional trade flows and raising supply chain concerns. Authorities monitor conditions for resumption.

Trump Warns Iran: ‘Massive Armada’ Deployed as Nuclear Talks Urged
U.S. President Trump warns Iran of a “massive armada” deployment, urging immediate nuclear negotiations to avoid military escalation. Regional tensions rise.

Rwanda Initiates Legal Action Against UK Over Terminated Asylum Deal
Rwanda seeks arbitration over unpaid funds from the UK’s canceled 2022 asylum agreement, escalating diplomatic tensions amid migration policy debates.

Sydney’s Microplastic Crisis: Pollution Triples in 3 Years, Report Warns
A new report reveals microplastic levels in Sydney’s waterways have tripled since 2022, raising environmental and public health concerns. Government initiatives aim to curb the crisis by 2028.

Egypt Mediates De-Escalation Efforts Between Iran and US in High-Stakes Calls
Egyptian FM holds talks with Iranian and US envoys to ease regional tensions, stressing diplomacy for nuclear deal and economic stability in critical week for Middle East relations.

MONUSCO Repatriates Former FDLR Fighters in Eastern DR Congo Peace Push
UN mission repatriates 34 Rwandans, including ex-FDLR fighters, as part of DR Congo-Rwanda peace efforts. Over 300 returned since 2025.

Greenland Stands Firm on Sovereignty Amid U.S. Negotiations
Greenland’s PM asserts sovereignty in U.S. talks, highlighting Arctic security concerns and European unity amid diplomatic tensions.

UK PM Starmer’s China Visit Strengthens Trade Ties in 2026
UK Prime Minister Keir Starmer’s 2026 China visit aims to deepen trade and investment ties, following $103.7B bilateral goods trade in 2025.

Xinjiang’s Khorgas Port Sets Cargo Record in 2025 Amid Trade Innovations
Khorgas Port in Xinjiang hits record 46M tons of cargo in 2025, driven by smart logistics reforms and surging new energy vehicle exports.