
Beijing Ducks Edge Shanxi Loongs in Nail-Biting 87-86 CBA Clash
The Beijing Ducks narrowly defeated the Shanxi Loongs 87-86 in a thrilling CBA matchup, maintaining their fourth-place standing. Key plays by Eugene German sealed the victory.

Qatar’s Sheikh Joaan Elected President of Olympic Council of Asia
Qatar’s Sheikh Joaan Bin Hamad Al Thani elected OCA president, emphasizing unity and youth empowerment in Asian sports during Tashkent assembly.

Sinner Extends Win Streak, Advances to Australian Open Quarterfinals
Jannik Sinner secures 18th straight win at Australian Open 2026, advancing to quarterfinals. Defending champion to face Ben Shelton in high-stakes rematch.

U.S. Exits Paris Climate Pact Again as China Advances Green Goals
As the U.S. formally exits the Paris Climate Agreement for the second time, China accelerates renewable energy investments, reshaping global climate dynamics in 2026.
UCAS Launches School of Space Exploration in 2026
UCAS establishes a cutting-edge School of Space Exploration in 2026, training future leaders through interdisciplinary programs and advanced research facilities.

EU Condemns Trump’s NATO Remarks, Greenland Tensions Escalate
European leaders denounce former U.S. President Trump’s NATO comments and military threats over Greenland, straining transatlantic relations in 2026.

China’s Industrial Profits Rebound in 2025 After Three-Year Slump
China’s large-scale industrial enterprises saw a 0.6% profit growth in 2025, ending a three-year decline, driven by policy support and manufacturing resilience.

Japan’s Early Election Sparks Political Strategy Debate
Japan’s snap election called by PM Takaichi raises questions about political strategy amid economic challenges and regional tensions. Analysis of domestic and international implications.

U.S. Military Buildup in Middle East Sparks Iranian Warning Amid Regional Tensions
U.S. deploys carrier group to Middle East amid rising tensions with Iran, prompting regional security concerns and market watchfulness in January 2026.

The ‘Kill Line’ Phenomenon: America’s Deepening Crisis of Resilience
Exploring the ‘kill line’ phenomenon in the U.S., where systemic fragility leaves millions one crisis away from collapse. Analysis of institutional erosion and its impact on social stability.

Xi Jinping, Finnish PM Orpo Strengthen Ties in Beijing Talks
Chinese President Xi Jinping and Finnish PM Petteri Orpo discussed enhanced cooperation in green tech and Arctic development during their Beijing meeting on January 27, 2026.

China Condemns Japan’s Taiwan Remarks, Urges Historical Reflection
China urges Japan to correct PM Takaichi’s remarks on Taiwan, stressing historical and legal facts of Taiwan’s return to China as part of the post-WWII international order.
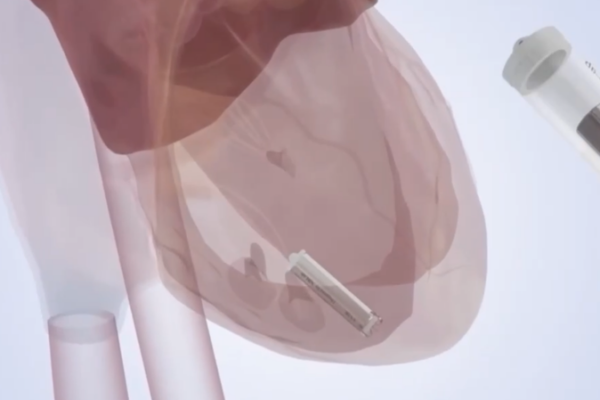
China Unveils Self-Powered Micro Pacemaker, Revolutionizing Cardiac Care
Chinese researchers develop self-powered micro pacemaker that could eliminate replacement surgeries, representing a major advance in implantable medical technology.

China Unveils 286-Strong Squad for Milano Cortina 2026 Winter Olympics
China announces 286-member Winter Olympics squad for 2026 Games, featuring defending champions Gu Ailing and Su Yiming alongside veterans and new talent.
Chinese Scientists Decode Genome of Protected Fish Species, Boost Conservation Hopes
Chinese researchers achieve breakthrough in mapping the genome of Bagarius rutilus, aiding conservation efforts for the endangered species.

Hamas, Israel Confirm Completion of Hostage Returns Under 2025 Ceasefire
Hamas states recovery of last Israeli hostage’s remains fulfills ceasefire terms as Israel confirms all captives returned from Gaza. Tensions persist over agreement compliance.

China Unveils AI-Driven Extreme Weather Forecasting System for 2026
China announces AI-powered weather forecasting system in 2026 to enhance extreme climate event predictions, boosting disaster preparedness.

US Announces Tariff Hike on South Korean Imports Amid Trade Tensions
The US plans to raise tariffs on select South Korean imports to 25%, prompting Seoul to seek urgent talks. Trade tensions escalate in early 2026.

Major Winter Storm Disrupts U.S., Impacts Felt Across Asia-Dependent Sectors
A historic U.S. winter storm disrupts global supply chains and diaspora travel plans, with significant implications for Asian economies and communities worldwide.

U.S. Border Chief to Exit Minneapolis Amid Shooting Fallout
U.S. Border Patrol Chief Gregory Bovino to leave Minneapolis after fatal shooting controversy, as Trump sends new envoy to oversee operations.













