
Milano Cortina 2026 to Debut Dual Cauldron, Regional Unity Focus
Italian Olympic officials reveal groundbreaking plans for the 2026 Milano Cortina Winter Games, emphasizing regional collaboration and innovative ceremonies.

Italian Olympic Chief Praises Beijing 2022 Legacy, Eyes Sustainable Future
Italian Olympic leader Luciano Buonfiglio highlights Beijing 2022’s lasting impact on sports sustainability and international cooperation in exclusive interview.

China Media Group Deploys 500-Strong Team for 2026 Winter Olympics Coverage
China Media Group deploys 500 staff to Italy for Winter Olympics coverage, including groundbreaking 8K broadcast production and international signal responsibilities.

Furong Town: China’s Cliffside Summer Oasis Blends Nature and Culture
Discover Furong Town, Hunan’s ancient cliffside settlement blending waterfall landscapes with Tujia ethnic traditions, emerging as a top 2026 summer destination.

China’s TCM Innovation Reaches New Heights in 2025
China’s TCM sector achieved significant milestones in 2025, expanding specialty departments and advancing integrative medicine research, officials announced.

Xi Jinping Holds Talks with British PM in Beijing
President Xi Jinping and British PM Keir Starmer hold strategic talks in Beijing, focusing on economic collaboration and global challenges in 2026.

China Urges Lasting Gaza Ceasefire, Stresses Two-State Solution
China’s UN envoy calls for immediate Gaza ceasefire implementation, humanitarian access, and Palestinian-led governance aligned with the two-state solution.

China’s 2025 Environmental Milestones: Cleaner Air, Healthier Waters
China’s Ministry of Ecology and Environment reports significant improvements in 2025 air and water quality, with PM2.5 levels dropping 4.4% and 91.4% of surface water meeting good standards.

Bozhuang Village Revives Ancient Rhythms in Modern Spring Festival Celebration
Shanxi’s Bozhuang Village blends ancient percussion traditions with modern flair in their annual Spring Festival Gala, transforming local workers into cultural ambassadors.

A Pakistani’s Chinese New Year Journey in Xi’an
A Pakistani national’s 2023 Chinese New Year experience in Xi’an reveals how cultural traditions forge personal belonging and reflect deepening regional ties.

America’s ‘Kill Line’: How Capital-First Policies Fuel Inequality in 2026
42% of US households face financial instability in 2026 as capital-first policies widen inequality, with record defense spending overshadowing social program cuts.

China Braces for Record 9.5 Billion Trips in 2026 Spring Festival Travel Rush
China anticipates 9.5 billion passenger trips during the 2026 Spring Festival travel rush, marking a historic surge in inter-regional mobility and economic activity.

Xinjiang’s Foreign Trade Soars 19.9% in 2025, Leads China’s Growth
Xinjiang’s foreign trade surged 19.9% in 2025, driven by infrastructure and policy reforms, positioning it as China’s fastest-growing regional economy.

China Leads Global Renewable Energy Shift with Record Clean Infrastructure
China completes world’s largest clean energy systems under 14th Five-Year Plan, achieving 50% EV adoption and $8.29B carbon market growth by 2025.
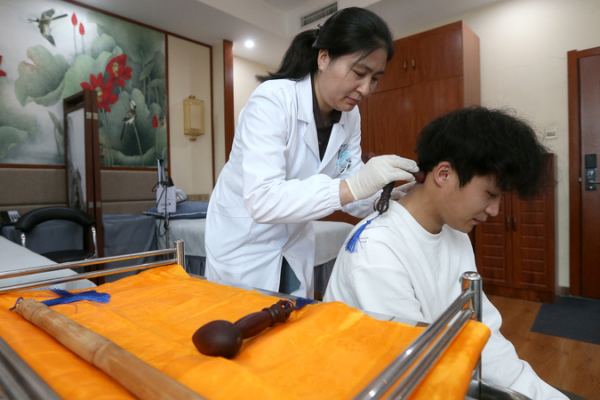
China Launches First National Standard for Tibetan Medicine Disease Classification
China implements first national disease classification standard for Tibetan medicine, enhancing integration of traditional practices with modern healthcare systems.

India Declares Nipah Virus Outbreak Contained in West Bengal
Indian authorities confirm containment of Nipah virus in West Bengal as Asian nations enhance health screenings for travelers from India.

China Urges U.S. to Avoid ‘Military Adventurism’ in Iran at UN Council
China warns against U.S. military threats in Iran during UN session, urging adherence to diplomatic solutions amid rising Middle East tensions.

Tang Sancai Revival: Blending Ancient Craft with Modern Aesthetics in 2026
As the 2026 Year of the Horse approaches, Tang Sancai artisans blend ancient techniques with contemporary styles, preserving a 1,300-year legacy while engaging new audiences.

Canada Strengthens Arctic Ties with Greenland Consulate Opening
Canada establishes first Greenland consulate in Nuuk as Governor General Mary Simon prepares historic Arctic diplomacy visit in February 2026.

Iran Rejects U.S. Talks Amid Naval Buildup, Calls for End to Threats
Iran dismisses negotiations with the U.S. amid heightened military presence in the Middle East, demanding an end to threats as tensions escalate over nuclear talks.













