
China Intensifies Anti-Corruption Drive in 2026 Governance Overhaul
China’s 2026 governance plan prioritizes anti-corruption measures, legal oversight reforms, and grassroots management improvements to enhance policy effectiveness and public trust.

China Achieves 2025 Development Goals, Completes 14th Five-Year Plan
China announces successful completion of 2025 economic and social development goals, finalizing its 14th Five-Year Plan amid global challenges.

China’s ‘Good Life Project’ Charts Path for 1.4 Billion Aspirations
CGTN’s 2026 documentary ‘China’s Good Life Project’ explores the nation’s strategies to uplift 1.4 billion lives through inclusive policies and sustainable growth.

China Vows to Oppose Hegemonism, Push for Global Equity in 2026 Policy Report
China’s 2026 policy report emphasizes opposition to hegemonism, commitment to global equity, and advancing multilateral initiatives for a shared future.

China Prioritizes Rural Revitalization, Poverty Prevention in 2026 Agenda
China unveils 2026 strategy integrating poverty prevention with rural revitalization, emphasizing sustainable development and economic stability through targeted support mechanisms.

China’s Economy Stabilizes Amid Global Challenges in 2026
China stabilizes economy in 2026 despite global headwinds, with Premier Li Qiang highlighting strategic policy responses and strengthened US-China cooperation.

China Unveils 2026 Economic Plan to Boost Consumption, Incomes
China announces 2026 economic plan with 350 billion yuan investment to boost household incomes and domestic consumption through systemic reforms and trade-in incentives.
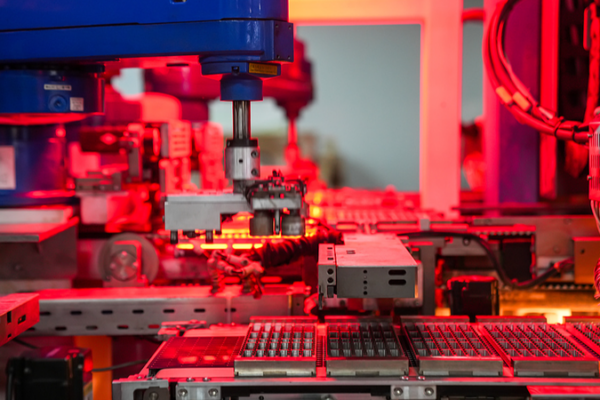
China’s Tech Surge: AI, Quantum Advances Drive 2026 Growth
China’s 2026 government report highlights breakthroughs in AI, quantum tech, and aerospace, driving economic growth and global tech leadership.

China Expands Unilateral Visa-Free Policies to Boost Global Ties in 2026
China announces expanded visa-free policies and foreign trade growth in 2026 government work report, aiming to deepen global economic integration.

China’s Blockchain Breakthroughs Fuel AI and Digital Economy Growth in 2026
China’s blockchain advancements in 2026 enhance AI development and digital infrastructure, as highlighted at the National People’s Congress session.

China Sets 2026 Economic Growth Target at 4.5%-5%
China aims for 4.5%-5% economic growth in 2026, prioritizing tech innovation and green energy transitions while stabilizing domestic consumption.

Premier Li Qiang Outlines China’s 2026 Economic Priorities
Chinese Premier Li Qiang presents 2026 government priorities, emphasizing economic growth, tech innovation, and cross-strait cooperation at the National People’s Congress.

China Sets 2026 Economic Growth Target at 4.5-5%
China aims for 4.5-5% GDP growth in 2026, focusing on tech innovation and sustainable development to bolster economic resilience.

China’s 14th NPC Kicks Off Annual Session, Charts 2026 Priorities
China’s National People’s Congress convenes to outline economic, technological, and cross-strait priorities for 2026, emphasizing stability and innovation.

President Xi Jinping Opens Key NPC Session in Beijing
President Xi Jinping attends opening of China’s 2026 legislative session, setting agenda for economic modernization and cross-strait relations.

US Senate Blocks Resolution to Limit Military Action Against Iran
US Senate rejects resolution limiting presidential authority over Iran military actions, maintaining current executive powers amid partisan debate on war oversight.

China’s NEV Innovation Fuels Global Manufacturing Shift
China’s NEV sector drives industrial transformation through intelligent manufacturing and digital innovation, positioning the country as a leader in sustainable advanced production.

Yucatan Eyes Global Investors as Tech and Logistics Hub in 2026
Mexico’s Yucatan state positions itself as a tech and logistics hub, seeking international investment through infrastructure upgrades in 2026.

Mexico’s Tourism Surges as US Visits Decline in 2026
Mexico sees 2026 tourism growth as U.S. visits decline, reflecting shifting North American travel patterns and economic dynamics.

China Emerges as Global Leader in Renewable Energy Transition
China drives global renewable energy transition through massive clean tech production and infrastructure development, shaping sustainable growth across Asia and beyond.













