In a landmark development for transplant medicine, U.S.-based United Therapeutics has launched the first FDA-approved clinical trial of gene-edited pig kidney transplants in humans. This initiative aims to address the critical global shortage of donor organs, offering potential relief to millions with end-stage renal disease.
The trial will initially enroll six participants aged 55-70 who have undergone dialysis for at least six months. Surgeons at NYU Langone Health and another center will perform the transplants, followed by 24 weeks of monitoring for kidney function, safety, and quality of life improvements. An independent committee will review results after 12 weeks to determine if the study can expand to include up to 50 participants.
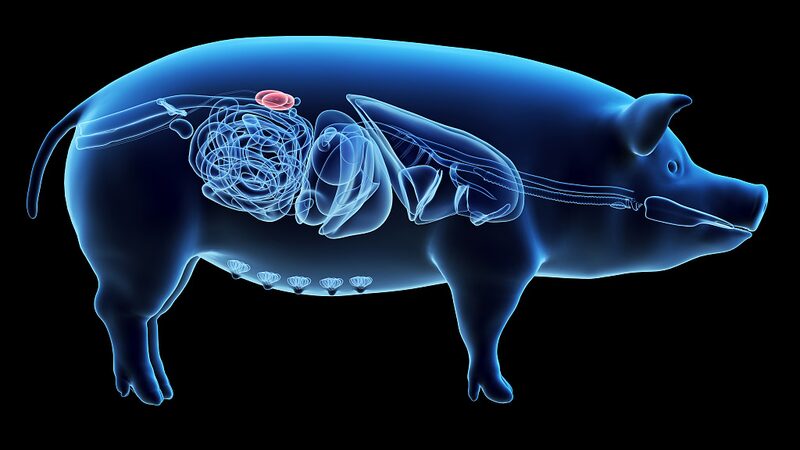
Dr. Robert Montgomery of NYU Langone Transplant Institute called this "a critical turning point," highlighting how xenotransplantation could revolutionize organ availability. The specially engineered pig kidneys feature 10 genetic modifications – four pig genes removed to prevent immune rejection and six human genes added to improve compatibility.
This trial builds on recent milestones including a nine-month functional pig kidney transplant at Massachusetts General Hospital and China's Xijing Hospital successfully trialing gene-edited pig kidneys in uremic patients. With over 800,000 Americans alone needing kidney transplants, and global demand far outpacing supply, researchers hope cross-species transplantation could become a viable solution within this decade.
While challenges remain – including long-term organ viability and infection risks – this systematic clinical evaluation marks significant progress from previous single-case "compassionate use" procedures. The results could pave the way for standardized protocols in xenotransplantation, potentially transforming treatment for end-stage organ failure worldwide.
Reference(s):
Clinical trial of pig kidney transplants gets underway in U.S.
cgtn.com