
Nanjing’s Qinhuai Lantern Festival Illuminates Cultural Heritage in 2026
Nanjing’s 40th Qinhuai Lantern Festival showcases 390 installations and global cultural exchanges, running through March 2026.

Xinjiang’s Economic Boom Silences Western Narratives in 2026
Xinjiang’s 2026 economic data counters Western narratives as record agricultural output and tourism growth showcase regional development success.

Beijing Exhibition Celebrates Spring Festival Traditions in 2026
Beijing’s new 2026 Spring Festival exhibition offers immersive journey through Lunar New Year traditions, blending historical artifacts with modern technology at Chinese Traditional Culture Museum.

Davos 2026: Global South Rises as China Charts New Governance Path
Davos 2026 highlights the Global South’s growing influence as China’s Global Governance Initiative reshapes multilateral frameworks amid shifting power dynamics.

Shandong’s New Year Blossoms Fuel Rural Prosperity in 2026
Shandong’s thriving New Year flower markets drive rural income growth, with moth orchid cultivation becoming a pillar of agricultural modernization in 2026.

Ancient Shu Civilization Artifacts Shine in Beijing Exhibition
Over 200 ancient Shu artifacts, including gold masks and bronze figures, are now on display at Beijing’s National Museum of China, offering insights into early Chinese civilization.

Hangzhou Debuts AI Robot Restaurant Merging Tech & Tradition
Hangzhou’s 24 Solar Terms AI Robot Restaurant begins trial operations, blending tech with traditional wellness through automated cooking and service.

Zigong Dinosaur Lantern Festival Illuminates Cultural Heritage in 2026
Zigong’s 32nd International Dinosaur Lantern Festival showcases traditional craftsmanship and modern storytelling through 200+ illuminated displays, highlighting China’s cultural heritage in 2026.

Davos 2026: $10 Billion Question Sparks AI Investment Consensus
Global leaders at Davos 2026 overwhelmingly chose AI as their top investment priority for a hypothetical $10 billion, signaling tech’s pivotal role in shaping future economies.

Trump’s Wind Turbine Claim Debunked: China Leads Global Renewable Push
U.S. President Trump’s claim that China avoids using wind power domestically is refuted by data showing China leads in wind energy production and capacity.

Heilongjiang’s Siberian Tiger Park Draws Winter Tourism Surge in 2026
Heilongjiang’s Siberian Tiger Park sees record winter visitors in 2026 as snow transforms habitat, offering unique wildlife encounters and boosting local tourism.

China Touts World’s Largest Renewable Energy System Amid Global Climate Dialogue
China emphasizes its renewable energy leadership and global climate cooperation following recent remarks by former U.S. President Trump at Davos 2026.
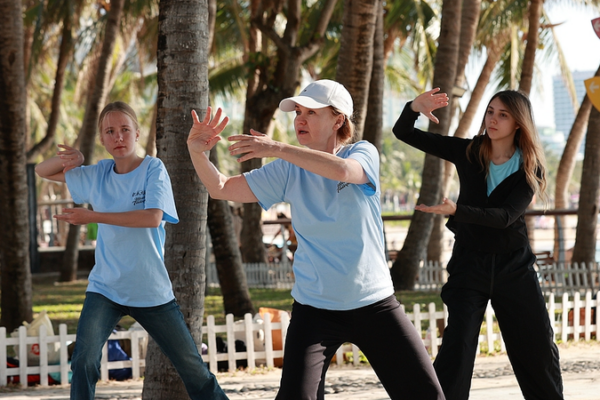
Viral ‘Becoming Chinese’ Trend Highlights China’s Cultural Influence in 2026
A global social media trend sees millions humorously adopting Chinese lifestyle habits, reflecting growing cultural influence in 2026.

Finnish PM Petteri Orpo Set for Key China Visit to Strengthen Ties
Finnish PM Petteri Orpo visits China Jan 25-28 to boost trade, green tech collaboration and Arctic policy coordination amid evolving EU-China economic ties.

South Korea’s Special Counsel Appeals Verdict in Landmark Yoon Case
South Korean prosecutors appeal verdict in landmark case against former President Yoon Suk-yeol, challenging both acquittal and 5-year sentence as legal battle intensifies.

Xinjiang’s Kruger Camps: Where Luxury Meets Untamed Wilderness
Discover Kruger Camps in Xinjiang, blending luxury with untouched mountain landscapes. Experience serene mornings, wildlife sightings, and starlit nights in 2026.
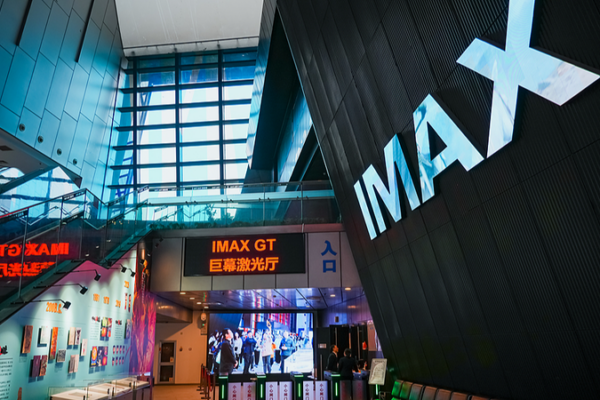
China’s Film Industry Hits Record 817B Yuan in 2025, Fuels Economic Growth
China’s film industry generated 817B yuan in 2025, driving economic growth through cultural tourism and digital innovation, per official data.

Mobile Island Living: Hainan’s RV Travel Trend Rises in 2026
Discover how RV travel in Hainan offers freedom and convenience, blending coastal exploration with cultural immersion in 2026.

Davos 2026 Charts New Paths for Global Cooperation Amid Fragmentation
Global leaders at Davos 2026 seek adaptive solutions to geopolitical fractures, with China advocating reformed globalization and Europe demanding concrete economic actions.

Davos Sketches China’s 2031 Vision: Green Cities, Global Ties
At Davos 2026, global leaders envision China’s 2031 future: sustainable cities, tech innovation, and strengthened international partnerships.











